Observational studies have associated metformin use with a decreased risk of lung cancer incidence in patients with type 2 diabetes, but the studies had important methodological shortcomings. The objective of this study was to determine whether metformin use is associated with a decreased risk of lung cancer in patients with type 2 diabetes, while avoiding previous biases.
Using the U.K. General Practice Research Database, we assembled a cohort of patients newly treated with oral hypoglycemic agents (OHAs) between 1988 and 2009. A nested case–control analysis was conducted, where case subjects with lung cancer occurring during follow-up were matched with up to 10 control subjects for age, sex, calendar time, and duration of follow-up. Conditional logistic regression was used to estimate adjusted rate ratios of lung cancer associated with ever use of metformin, along with measures of duration and cumulative dose. Models were adjusted for potential confounders, which included smoking.
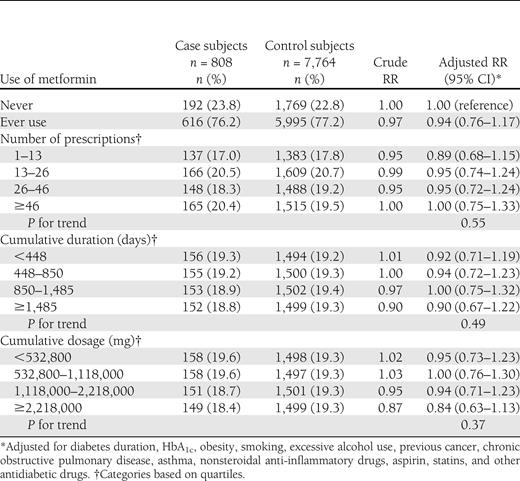
The cohort included 115,923 new users of OHAs, with 1,061 patients diagnosed with lung cancer during follow-up (rate 2.0/1,000 person-years). Metformin use was not associated with a decreased rate of lung cancer (rate ratio 0.94 [95% CI 0.76–1.17]). No dose-response was observed by number of prescriptions received, cumulative duration of use, and dose.
Metformin use is not associated with a decreased risk of lung cancer in patients with type 2 diabetes. The decreased risk reported in other observational studies is likely due to bias from methodological shortcomings. Nonetheless, greater consideration should be given to clarify inconsistencies between experimental models and population studies.
Metformin, a biguanide derivative, is considered a first-line treatment in patients with type 2 diabetes. In addition to controlling glycemic levels and reducing the risk of diabetes complications, several observational studies have reported that its use is associated with a decreased risk of cancer overall and across several specific cancer sites (1–7). With respect to lung cancer, only three observational studies have investigated that outcome (8–10). In one study, metformin use was associated with a 45% decreased risk of lung cancer (hazard ratio 0.55 [95% CI 0.37–0.82]) (9), whereas in the two other studies, no statistically significant association was found (8,10). However, these observational studies had several methodological shortcomings. Lung cancer was a secondary outcome in two of these studies, and thus, the findings may have been partly due to chance as a result of multiple comparisons (8,10). Furthermore, the method of the cohort selection was biased in one study (10), and exposure misclassifications led to immortal time bias in another study (9). Because of these methodological shortcomings, as well as insufficient follow-up in some of these studies, the relationship between metformin and lung cancer incidence remains unclear.
Laboratory studies (1,5–7,11) have shown cytostatic or cytotoxic effects of biguanides in various models and have provided evidence for biologic plausibility for an antineoplastic effect of metformin. For example, the drug has been shown to act systemically to reduce insulin levels if they are elevated at baseline (as is often the case in type 2 diabetes), and this may reduce proliferation of the subset of cancers that are growth-stimulated by insulin. Furthermore, by acting directly on cancer cells or on cells at risk for transformation, metformin and other biguanides can impair mitochondrial ATP production, leading to the activation of liver kinase B1 (LKB1)–AMP-activated protein kinase (AMPK) signaling, resulting in a decrease in protein synthesis and lipid synthesis via inhibition of mammalian target of rapamycin and fatty acid synthase, respectively.
Metformin may also have additional proposed mechanisms of action, and there is increasing interest in the hypothesis that metformin has utility in cancer prevention and/or treatment. Indeed, metformin inhibited tobacco carcinogen–induced lung cancer in an animal model (12), but to date, population-based studies conducted to evaluate the association between metformin and lung cancer have produced conflicting results due to the presence of several important biases. Given the time-related biases in previous observational studies, we conducted a large population-based study specifically designed to avoid these methodologic shortcomings to investigate the association between the use of metformin and the risk of lung cancer in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Data source
We used the U.K. General Practice Research database (GPRD), the world’s largest computerized database, representing the primary care longitudinal records of more than 11 million patients from across the U.K. The GPRD is representative of the U.K. general population, with age and sex distributions comparable to those reported by the U.K. National Population Census (13). All information collected in the GPRD has been subjected to validation studies and been proven to contain consistent and high-quality data (14). The study protocol was approved by the independent scientific advisory committee of the GPRD and the research ethics committee of the Jewish General Hospital, Montreal, Quebec, Canada.
Study cohort
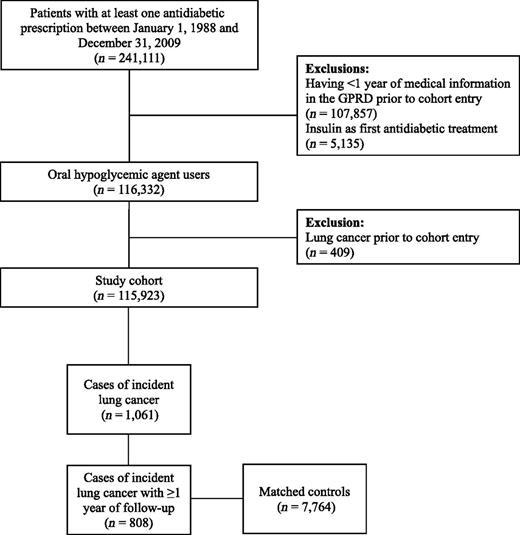
Within the GPRD population, we assembled a cohort of all patients, aged at least 40 years, who had received at least one antidiabetic prescription between 1 January 1988 and 31 December 2009. Cohort entry was defined as the date of a first prescription for an oral hypoglycemic agent (OHA) during this period. All patients included in the study were from up-to-standard general medical practices, thus meeting GPRD research quality standards, and were required to have at least 1 year of medical history in the GPRD before their cohort entry. Patients who received insulin as their first antidiabetic treatment were not included because they likely represented patients with type 1 diabetes or patients with advanced type 2 diabetes; however, patients who eventually required insulin during follow-up were retained. Finally, patients diagnosed with lung cancer at any time before cohort entry were excluded. All patients were monitored until a first-ever diagnosis of lung cancer, death from any cause, end of registration with a general practice, or end of the study period (31 December 2009), whichever came first.
Case and control subject selection
A nested case–control analysis was conducted within the defined cohort. All incident cases of lung cancer occurring during follow-up were identified on the basis of Read diagnostic codes, which is the standard clinical terminology system used in general practice in the U.K. (15). The date of each case subject’s lung cancer diagnosis was defined as the index date. For the purposes of the analyses, only case subjects with at least 1 year of follow-up were retained to consider a latency period. Up to 10 control subjects, randomly selected from the case subject’s risk set, were matched to each patient on year of birth (age), sex, calendar year of cohort entry, and duration of follow-up. To avoid excluding patients, the matching criteria were relaxed for four lung cancer patients. Three individuals were matched with a control subject who had the same year of cohort entry ± 1 year, and one patient was matched to control subjects with a year of birth ± 3 years and the year of cohort entry ± 2 years. Control subjects were assigned the same index date as the patients, thus ensuring that case subjects and matched control subjects had equal duration of follow-up before the index date. By definition, all control subjects were alive, not previously diagnosed with lung cancer, and were registered with a general practice when matched to a given case subject. Cancer diagnoses, including lung cancer, have shown high validity in the GPRD, with sensitivities and positive predictive values exceeding 90% (16–19), resulting in case ascertainment rates comparable to U.K. cancer registries (20).
Exposure assessment
For both case and control subjects, we obtained information on all antidiabetic agents prescribed between cohort entry and the index date. Exposures initiated in the year before the index date were excluded from the analysis to account for a latency time window, because these are unlikely to be associated with the outcome. The primary exposure definition was ever use of metformin, defined as receiving at least one prescription between cohort entry and the year before the index date.
In secondary exposure definitions, we considered whether a dose–response relationship existed between the use of metformin and lung cancer. Therefore, among patients deemed to have ever used metformin in the primary exposure definition, we investigated whether lung cancer risk varied with the total number of prescriptions received, cumulative duration, and cumulative dose. The total number of metformin prescriptions was tabulated by summing all metformin prescriptions received between cohort entry and index date. Cumulative duration was calculated by summing the prescribed duration associated with each metformin prescription received between cohort entry and index date, and cumulative dose was computed by multiplying the daily dose of each metformin prescription by its specified prescription duration and adding these prescription-specific values across all prescriptions received by a given patient between cohort entry and index date. All three dose–response variables were categorized in quartiles, based on the distribution of use in the control subjects.
Potential confounders
The risk estimates were adjusted for comorbid clinical conditions and exposures known to be associated with lung cancer that might also influence the choice of antidiabetic therapy. These conditions and exposures were measured at any time from at least 1 year before cohort entry up to 1 year before the index date. Thus, the following potential confounders were considered: smoking status (ever, never, or unknown), BMI (≥30 vs. <30 kg/m2), excessive alcohol use, last recorded glycated hemoglobin A1c (HbA1c) at least 1 year before index date, diabetes duration before cohort selection (defined as a diagnosis of type 2 diabetes or an HbA1c level >7.0%, whichever appeared first in the medical record), chronic obstructive pulmonary disease, and asthma (18), previous cancer (other than nonmelanoma skin cancer), and ever use of statins, aspirins, nonsteroidal anti-inflammatory drugs, and other antidiabetic agents that were individually adjusted for in the model, including metformin, sulfonylureas, thiazolidinedione, insulins, and others, consisting of meglitinides, dipeptidyl peptidase-4 inhibitors, α-glucosidase inhibitors, glucagon-like peptide analogs, and guar gum.
Statistical analysis
The characteristics of the case subjects and matched control subjects were summarized using descriptive statistics. The overall lung cancer incidence rate with 95% CI based on a Poisson distribution was calculated by dividing the total number of patients with incident lung cancer occurring during follow-up by the total number of person-years of follow-up.
Conditional logistic regression was used to estimate rate ratios (RRs) along with 95% CIs of lung cancer associated with the use of metformin. The regression models were conditioned on the four matching factors (age, sex, calendar year of cohort entry, and duration of follow-up) and adjusted for the potential confounders listed above. In the primary analysis, we evaluated whether ever use of metformin, when compared with never use, was associated with a decreased risk of lung cancer.
We also conducted two secondary analyses. In the first analysis, we determined whether there was a dose–response between the use of metformin and lung cancer in number of prescriptions, cumulative duration of use, and cumulative dose. Linear trend was assessed by entering these dose–response variables in the conditional logistic models as continuous variables. In the second analysis, we stratified case and control subjects on smoking status to determine whether smoking was an effect modifier of the metformin-lung cancer association. All analyses were conducted with SAS 9.2 software (SAS Institute, Cary, NC).
Sensitivity analyses
We conducted three sensitivity analyses to assess the robustness of the findings. Initially, all analyses were restricted to case and matched control subjects with at least 1 year of follow-up and excluded antidiabetic medications initiated during the year before the index date to consider a latency time window. Thus, the first sensitivity analysis consisted of repeating the analyses by using latency time windows of 6 months and 2 years. In the second sensitivity analysis, we assessed potential misclassification of exposure by redefining ever use of metformin as receiving at least three prescriptions within a 12-month period, thus minimizing the inclusion of patients who may not have been regular users or who used these drugs sporadically. Finally, in the third sensitivity analysis to assess the effect of adjusting for variables potentially on the casual pathway, we repeated the analysis, adjusting for the potential confounders measured at cohort entry.
RESULTS
A total of 115,923 patients (55.2% men) newly treated with OHAs met the study inclusion criteria (Fig. 1). Mean age was 64.1 (SD 12.0) years, and the median HbA1c was 8.2% at cohort entry. With respect to OHAs received at cohort entry, 67.4% of patients received metformin monotherapy, 29.6% received sulfonylureas monotherapy, 1.3% received other OHAs in monotherapy, and 1.7% were taking a combination of at least two OHAs.
The mean follow-up was 5.6 (SD 3.6) years, generating 528,356 person-years of follow-up. During this time, 1,061 patients were diagnosed with lung cancer, resulting in an overall lung cancer rate of 2.0/1,000 person-years (95% CI 1.9–2.1). The nested case-control analysis was restricted to the 808 case subjects and 7,765 matched control subjects with at least 1 year of follow-up.
As reported in Table 1, case subjects and matched control subjects were similar on several characteristics, such as duration of diabetes before cohort entry, HbA1c, BMI, and ever use of nonsteroidal anti-inflammatory drugs. As expected, the prevalence of smoking was higher in case subjects than in matched control subjects (85.2 vs. 60.0%, respectively). Furthermore, case subjects were more likely than control subjects to have had a history of chronic obstructive pulmonary disease, to have had a history of asthma, to have used alcohol excessively, and to have ever used aspirin and statins (Table 1).
Table 2 presents the results of the primary analysis. Overall, the use of metformin was not associated with a decrease rate of lung cancer (adjusted RR 0.94 [95% CI 0.76–1.17]). In secondary analyses, no dose-response was observed in number of metformin prescriptions received, cumulative duration, and cumulative dose, with all adjusted RRs around the null value (Table 2).
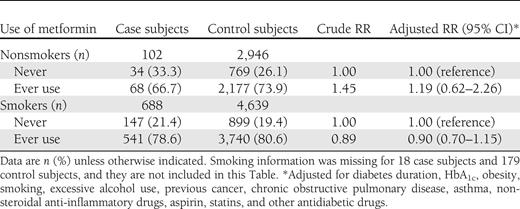
When case and control subjects were stratified on a smoking status, no effect modification was observed with respect to this variable (Table 3). In nonsmokers, ever use of metformin was not associated with a decreased rate of lung cancer (adjusted RR 1.19 [95% CI 0.62–2.26]). Likewise, no association was observed in smokers (0.90 [0.70–1.15]; Table 3).
Sensitivity analyses
In the first sensitivity analysis, varying the latency time window to 6 months and 2 years yielded results consistent with those of the primary analysis (adjusted RR 1.02 [95% CI 0.83–1.26] and 1.02 [0.80–1.29], respectively). In the second analysis, we assessed the effect of potential exposure misclassification by redefining ever use of metformin as receiving at least three prescriptions within a 12-month period. This analysis yielded null results, consistent with those of the primary analysis (0.97 [0.80–1.17]). Finally, adjusting for potential confounders at baseline did not materially change the results (0.97 [0.78–1.20]).
CONCLUSIONS
The results of this large population-based study indicate that the use of metformin is not associated with a decreased risk of lung cancer in patients with type 2 diabetes. These results remained unchanged in secondary analyses, which considered dose-response, by smoking status, as well as in several sensitivity analyses. As such, our findings do not support laboratory models that focused on the direct and indirect effect of metformin on lung cancer and tumor proliferation (11,12). Although laboratory data have provided evidence for plausible mechanisms of action of biguanides that may reduce cancer risk and/or improve cancer prognosis, such plausibility of course does not necessarily demonstrate that metformin has clinical antineoplastic activity. The models do not fully recapitulate the clinical situation in many respects, but one obvious area for future investigation concerns pharmacokinetics and drug exposure levels in lungs clinically as compared with in the models.
The findings of this study are comparable to those observed by Ferrara et al. (8), where ever use of metformin, compared with never use, was not associated with a decreased risk of lung cancer (hazard ratio 1.0 [95% CI 0.8–1.1]). However our results contrast sharply with those published by Lai et al. (9). In that study, ever use of metformin was associated with a significant decreased risk of lung cancer (0.55 [0.37–0.88]) (9). Interestingly, similar risk reductions were observed in that study with other antidiabetic treatments, such as thiazolidinediones (0.55 [0.32–0.94]) and α-glucosidase inhibitors (0.61 [0.38–0.98]), while null results were observed for insulin (1.00 [0.68–1.45]) and sulfonylureas (1.27 [0.75–2.15]) (9). Such impressive risk reductions are likely due to immortal time bias, a bias that is introduced with time-fixed analyses that misclassify unexposed person-time as exposed (21,22).
The current study had a number of strengths. First, our study avoided immortal time bias by using a design and analysis that inherently considered exposure to metformin as time-dependent (21,22). Second, we were able to assemble a large cohort of patients with type 2 diabetes with a significant number of patients with lung cancer. Third, data are prospectively collected in the GPRD and thus recall bias is avoided. Fourth, the GPRD records information on a number of potential confounders, such as smoking, BMI, and HbA1c levels, which are often absent in administrative databases. Finally, we adjusted the models for HbA1c, duration of diabetes before cohort entry (i.e., duration of nontreated diabetes), and matched case and control subjects on duration of follow-up (i.e., duration of treated diabetes). We believe that all efforts went into controlling for the effects of diabetes and its severity, which may be independently associated with an increased risk of lung cancer (18).
This study also has some limitations. Although the GPRD contains information on variables such as smoking, which is perhaps the most important potential confounder in this study, the database lacks information on family history of lung cancer, race, level of physical activity, diet, past lung biopsies, bronchoscopies, computed tomography scans, and other hospital procedures related to lung cancer (23). Thus, residual confounding due to unmeasured or incompletely measured covariates may still be present, although these unmeasured variables are not strongly associated with the outcome and thus are unlikely to have affected the validity of the results (24).
Another limitation of the GPRD is the lack of information on compliance with the prescribed treatment. The GPRD only contains information on prescriptions written by general practitioners, and therefore, whether prescriptions were actually filled or taken as indicated by patients is unknown. Such exposure misclassification would bias the RRs toward the null. However, the results of our sensitivity analysis requiring at least three prescriptions within a 12-month period suggests that this misclassification was likely minimal. Finally, although cancer diagnoses have been shown to be well recorded in the GPRD (16–19), the database does not contain specific information on tumor grade and stage, and thus, it was not possible to stratify the patients by using these parameters.
In summary, this large population-based study provides evidence that the use of metformin is not associated with a decreased risk of lung cancer in patients with type 2 diabetes. This finding remained consistent after conducting several secondary and sensitivity analyses. Our observations, however, do not detract from the plausibility of the mechanisms of antineoplastic action of biguanides demonstrated by laboratory models (1–7,25,26) but suggest that these mechanisms do not operate clinically, at least at the conventional doses used in the treatment of type 2 diabetes. Therefore, further translational research, including careful attention to drug exposure levels in relevant organs, is suggested before launching large-scale randomized controlled trials of metformin for proposed indications in oncology.
Acknowledgments
This research was partly funded by an infrastructure grant from the Canadian Institutes of Health Research and the Canadian Foundation for Innovation. S.S. is the recipient of the James McGill Chair, and L.A. is the recipient of a Chercheur-Boursier Award from the Fonds de la recherche en santé du Québec.
No potential conflicts of interest relevant to this article were reported.
B.B.S. contributed to the study concept and design, to analysis and interpretation of data, to drafting of the manuscript, and to critical revision of the manuscript for important intellectual content. L.A. contributed to the study concept and design, to analysis and interpretation of data, and to critical revision of the manuscript for important intellectual content. H.Y. and M.N.P. contributed to analysis and interpretation of data and to critical revision of the manuscript for important intellectual content. S.S. supervised the study and contributed to the study concept and design, to analysis and interpretation of data, and to critical revision of the manuscript for important intellectual content. S.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.