Alginate (Alg)-encapsulated porcine islet cell grafts are developed to overcome limitations of human islet transplantation. They can generate functional implants in animals when prepared from fetal, perinatal, and adult pancreases. Implants have not yet been examined for efficacy to establish sustained, metabolically adequate functional β-cell mass (FBM) in comparison with human islet cells. This study in immune-compromised mice demonstrates that subcutaneous implants of Alg-encapsulated porcine prenatal islet cells with 4 × 105 β-cells form, over 10 weeks, a FBM that results in glucose-induced plasma C-peptide >2 ng/mL and metabolic control over the following 10 weeks, with higher efficiency than nonencapsulated, while failing in peritoneum. This intracapsular FBM formation involves β-cell replication, increasing number fourfold, and maturation toward human adult β-cells. Subcutaneous Alg-encapsulated human islet cells with similar β-cell number establish implants with plasma C-peptide >2 ng/mL for the first 10 weeks, with nonencapsulated cells failing; their β-cells do not replicate but progressively die (>70%), explaining C-peptide decline and insufficient metabolic control. An Alg matrix thus helps establish β-cell functions in subcutis. It allows formation of sustained metabolically adequate FBM by immature porcine β-cells with proliferative activity but not by human adult islet cells. These findings define conditions for evaluating its immune-protecting properties.
Introduction
β-Cell replacement can cure type 1 diabetes. Intraportal implants of human pancreatic islet cells can restore endogenous insulin production in patients with depleted β-cell mass (1). Shortage in donor organs and variable quality of islet cell isolates limit this form of therapy. This led to efforts on alternative sources that allow mass production of grafts that form insulin-producing implants in rodents and offer a perspective for clinical translation, in particular, porcine pancreases for isolation of β-cell preparations, and human stem cells for in vitro derivation of pancreatic endoderm and subsequent stages in β-cell differentiation (2,3). Both strategies include combinations with encapsulation methods in order to protect implants against immune reactivity and, hence, avoid the need for continuous immune suppression (4–6).
Use of encapsulated grafts requires implants outside a vital organ such as liver or kidney, preferably in a confined site where the implant can be entirely retrieved when needed for safety. It is thus far unknown in which extraportal site a metabolically adequate β-cell replacement can be achieved in patients, with at least the same outcome as intraportal implants (1). Confined sites offer the potential to retrieve representative samples for directly assessing functional β-cell mass (FBM) in the implants and its relationship with in vivo metabolic outcome. This analysis can determine dependency of outcome on initial graft characteristics, such as volume, cell number, and composition, which has significance for clinical dosing and applicability. It also allows comparison of β-cells formed by alternative sources with those in human pancreatic islet isolates. Establishment and maintenance of a metabolically adequate FBM are a key goal in β-cell replacement. Studies in animal models can examine whether and to what extent this goal is met by alternative cell therapy products.
At present there is not, in our opinion, one animal model in which all expectations of encapsulated implants of large-scale cell sources can be addressed. The current study uses immune-compromised mice to examine whether a FBM can be established and maintained within subcutaneous alginate (Alg) microcapsules that contain islet cells purified from dispersed prenatal porcine organs (7). FBM of implants is determined by direct assessment of its main components, i.e., the number of β-cells and their functional state (8). Both parameters are compared with their values in the graft and related to the plasma porcine C-peptide (p-C-peptide) levels, an indirect FBM marker. The combined FBM markers serve to identify implant characteristics that control glycemia. Several studies have used plasma p-C-peptide levels to demonstrate function of Alg-encapsulated porcine β-cell implants in small and large animals (9–13), but little insight exists on their relationship with the initial β-cell dose, with the course of the implanted β-cell number and function with time, and with the associated metabolic control. This information is, however, important for identifying the potential of these grafts for β-cell replacement and its site dependency. We addressed this question in immune-compromised recipients, as the goal is to first evaluate functional capacity of implants in absence of an immune reactivity. Data can then serve as reference when assessing outcome in immune-competent recipients with or without associated treatment. To evaluate clinical relevance of data, we compared FBM resulting from Alg-encapsulated porcine prenatal islet cell (Alg-pp-IC) grafts with that from Alg-encapsulated human islet cell (Alg-hu-IC) grafts containing the same β-cell number at start.
Research Design and Methods
Preparation of Alginate-Encapsulated Pancreatic Islet Cell Grafts
The term “graft” is used for cell preparations that will be implanted and the term “implant” for their occurrence in recipients or following retrieval. Alg-encapsulated grafts (652 ± 48 µm diameter) (Fig. 1A) were prepared from prenatal porcine islet cells and from adult human islet cells, using conditions previously reported for human islet cell grafts (14): suspension of 20 × 106 cells/mL in 2% ultrapure high mannuronic acid (high M) Alg (Nova Matrix, Sandvika, Norway) with 50 mmol/L CaCl2 and 1 mmol/L BaCl2 as gelling solution. Nonencapsulated cells were studied in parallel.
Alg-pp-IC (106 cells per recipient) before transplantation (A and B) and at week 20 following transplantation in the subcutis (C–E) or in the peritoneal cavity (F). Alg-hu-IC (3.106 cells per recipient) before transplantation (G) and at week 17 following transplantation in the subcutis (H). Immune labeling for insulin-positive (INS) (red) and glucagon-positive (GCG) (green) cells or for synaptophysin-positive (SYN) (red) and vimentin-positive (VIM) (green) cells; nuclei by DAPI (blue). Immunostaining was performed with guinea pig anti-insulin and mouse anti-glucagon antibodies (1:2,000) (produced in-house), mouse anti-synaptophysin antibodies (1:100) (Dako, Glostrup, Denmark), and rabbit anti-vimentin antibodies (1:1,000) (Abcam, Cambridge, U.K.) using Alexa Fluor 647, Alexa Fluor 488, and Cy3-conjugated donkey antibodies (1/500) (Jackson ImmunoResearch, West Grove, PA) as secondary antibodies. Scale bars = 50 µm.
Alg-pp-IC (106 cells per recipient) before transplantation (A and B) and at week 20 following transplantation in the subcutis (C–E) or in the peritoneal cavity (F). Alg-hu-IC (3.106 cells per recipient) before transplantation (G) and at week 17 following transplantation in the subcutis (H). Immune labeling for insulin-positive (INS) (red) and glucagon-positive (GCG) (green) cells or for synaptophysin-positive (SYN) (red) and vimentin-positive (VIM) (green) cells; nuclei by DAPI (blue). Immunostaining was performed with guinea pig anti-insulin and mouse anti-glucagon antibodies (1:2,000) (produced in-house), mouse anti-synaptophysin antibodies (1:100) (Dako, Glostrup, Denmark), and rabbit anti-vimentin antibodies (1:1,000) (Abcam, Cambridge, U.K.) using Alexa Fluor 647, Alexa Fluor 488, and Cy3-conjugated donkey antibodies (1/500) (Jackson ImmunoResearch, West Grove, PA) as secondary antibodies. Scale bars = 50 µm.
Prenatal porcine islet cell aggregates were prepared by Beta Cell NV according to previously described isolation and culture conditions (7). Organs were retrieved at gestation day 110–115, dissociated by collagenase, and dispersed in calcium-free medium. Single endocrine cells were purified and cultured in a serum-free Ham’s F10 medium. After 6 days of culture, aggregates were microencapsulated and cultured overnight before transplantation (Fig. 1A and B).
Adult human islet cell aggregates were prepared by the Beta Cell Bank at University Hospital Brussels, which operates in a clinical islet cell transplant program (15,16). Donor organs were allocated by Eurotransplant and processed to quality-controlled cultured islet cell isolates; when quantitatively insufficient for implants in patients, they can be used for research projects if fulfilling Eurotransplant guidelines. These cultured human islet cell preparations have been shown to exhibit glucose-responsive secretory and synthetic activities with first-phase insulin release in perifusion and to establish functional implants in patients and in immune-compromised rodents (14–16). This supports their use for in vitro and in vivo comparison with human stem cell–generated β-cells formed in implants (17) and with porcine β-cells that have differentiated in implants as in the current study.
Transplantation of (Non)encapsulated Islet Cell Grafts in Immune-Compromised Mice
After overnight culture, cell number and composition were determined in samples of (non)encapsulated cell preparations using the nuclear count assay (NucleoCounter YC-100; ChemoMetec, Allerød, Denmark) and immunocytochemistry (minimally 1,000 cells counted) (14,16). Capsule number was counted before dissolving the Alg matrix with lyase (0.5 mg/mL) (Sigma-Aldrich, St. Louis, MO) and performing the nuclear count. Cell composition and insulin content were determined on washed capsules and nonencapsulated aggregates (18). Grafts with defined cell number were implanted in normoglycemic male immune-compromised NOD.CB17-Prkdcscid/J mice (7–8 weeks old; Charles River, Saint-Germain-Nuelles, France). Surgery occurred under general anesthesia (10 mg/kg xylazine [Bayer, Leverkusen, Germany] and 100 mg/kg Ketamine 1000 [Ceva Santé Animale, Brussels, Belgium]). Recipients were followed up to 20 weeks posttransplantation (PT). The studies were approved by the ethics committee for animal experimentation of Brussels Free University; manipulations were carried out in accordance with European Community Council Directive 86/609/EEC.
Alg-pp-IC grafts containing 3 × 106 or 106 cells were injected under the skin (total area 4–6 cm2 per 106 cells) or in the peritoneal cavity. Nonencapsulated prenatal porcine islet cell grafts were loaded on GELFOAM (Pfizer, New York, NY) before insertion under the skin. The prenatal porcine islet cell grafts contained 1.2 × 106 or 4 × 105 β-cells at 42 ± 4% purity; other cell types were mainly endocrine (27 ± 3% α-cells and 11 ± 2% δ-cells). (Non)encapsulated human islet cell grafts containing 2 to 4 × 106 cells were also implanted under the skin; human islet cells were used after 25 ± 13 days of culture and consisted of 106 β-cells at 40 ± 9% purity, with 15 ± 4% α-cells, 5 ± 1% δ-cells, and 38 ± 15% nonendocrine duct cells.
Tail vein blood was collected to determine glycemia and plasma C-peptide (basal [2 h fasting] and glucose stimulated [15 min after intraperitoneal injection of 3 g/kg body wt]). ELISA was used to measure porcine (Mercodia, Uppsala, Sweden) and mouse C-peptide (m-C-peptide) (Crystal Chem, Elk Grove Village, IL), and trefoil-type time-resolved fluorescence immunoassay for human C-peptide (hu-C-peptide) (17).
Analysis of Encapsulated Implants
Encapsulated implants and pancreases were retrieved for analysis at PT week 1, 5, 10, or 20. Capsules were isolated from the tissue by mild collagenase treatment (1 mg/mL) (type XI; Sigma-Aldrich), counted (TE2000E Nikon microscope with manual counting program NIS-Elements AR v4.20 software), and distributed to determine cell composition and β-cell functions. Cell numbers were determined after treatment with lyase.
For analysis of cellular composition, samples were fixed in Bouin solution, embedded in paraffin, sectioned, and treated with citrate (Scytek Laboratories, Logan, UT), protease (Sigma-Aldrich), or R-Universal (Aptum Biologics, Southampton, U.K.). Antibodies to identify (non)endocrine cells are listed in the legend of Fig. 1; rabbit anti-Ki67 antibody (Acris, Herford, Germany) was used to mark cells in proliferative activity. Digital images were generated on an Axioplan 2 (Carl Zeiss, Oberkochen, Germany) microscope with an Orca-R2 camera (Hamamatsu Photonics) and analyzed with SmartCapture 3 software (DSUK, Cambridge, U.K.). Electron microscopy was used to assess density of secretory vesicles in β-cells and their degree of maturation (17).
For analysis of β-cell functions, defined numbers of capsules were examined for insulin secretory responses and for insulin synthetic and storage capacity as previously described (16). Insulin secretion was measured in perifusion at different glucose concentrations in the absence or presence of 10 nmol/L glucagon and insulin synthesis during 2-h static incubation at low and high glucose concentration using L-[3,5-3H]-tyrosine (PerkinElmer, Boston, MA) as tracer for newly synthesized proteins and insulin; the amount of [3H]-labeled (pro)insulin was determined after immunoprecipitation. Cellular insulin content was assayed after dissolving the Alg matrix.
Statistical Analysis
Results are expressed as means ± SD. Statistical analysis was carried out using Prism 5.0 (GraphPad Software, San Diego, CA). Groups were compared using Student t test or one-way ANOVA with Tukey post hoc test (statistically significant at P < 0.05).
Results
Subcutaneous Implants of Alginate-Encapsulated Porcine Prenatal Pancreatic Endocrine Cells Establish Sustained FBM With Metabolic Control in Mice
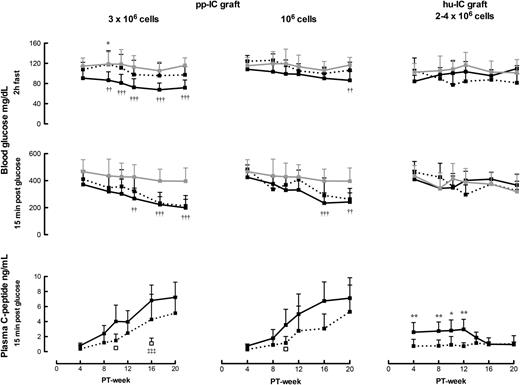
Plasma p-C-peptide >0.5 ng/mL at min 15 following glucose injection was used as a marker for hormone-releasing porcine β-cells. It was not detected in control mice (<0.01 ng/mL detection limit) but was present at PT week 4 in all recipients of subcutaneous Alg-pp-IC (3 × 106 cells per recipient). Levels were followed over 20 weeks to identify a loss or increase in FBM. They progressively increased in all animals up to PT week 16 and then leveled off at 3–10 ng/mL, on average eightfold higher than at PT week 4 (Fig. 2). This course was similar for nonencapsulated subcutaneous implants (P > 0.05). It was also achieved by subcutaneous Alg-pp-IC with threefold lower initial cell dose (Fig. 2), reaching, at PT week 19–20, plasma p-C-peptide levels of 1.2 ± 0.8 ng/mL basal and 6.2 ± 2.6 ng/mL 15-min post–intraperitoneal glucose (n = 17–P < 0.001). When injected intraperitoneally, plasma p-C-peptide remained low or undetectable (Fig. 2).
Blood glucose and plasma p-C-peptide levels following subcutaneous implantation of Alg-pp-IC grafts containing 3 × 106 cells (n = 12–19) (solid line, left panel) or 106 cells (n = 19–30) (solid line, middle panel). Data from nonencapsulated implants in the subcutis are represented by the dotted curves (n = 3–9) and those from encapsulated implants in the peritoneal cavity by the open squares (n = 3). The right panel shows blood glucose levels and plasma hu-C-peptide levels following subcutaneous implantation of human islet cell (hu-IC) grafts containing 2–4 × 106 cells (solid lines for Alg-encapsulated and dotted lines for nonencapsulated preparations). Data from nontransplanted control mice are illustrated by the gray lines. All data are expressed as means ± SD. Significance of differences was calculated by one-way ANOVA with Tukey post hoc test, comparing nonencapsulated and encapsulated implants (*P < 0.05, **P < 0.01), encapsulated implants and nontransplanted controls (††P < 0.01, †††P < 0.001), and encapsulated implants in subcutis and peritoneal cavity (‡‡‡P < 0.001).
Blood glucose and plasma p-C-peptide levels following subcutaneous implantation of Alg-pp-IC grafts containing 3 × 106 cells (n = 12–19) (solid line, left panel) or 106 cells (n = 19–30) (solid line, middle panel). Data from nonencapsulated implants in the subcutis are represented by the dotted curves (n = 3–9) and those from encapsulated implants in the peritoneal cavity by the open squares (n = 3). The right panel shows blood glucose levels and plasma hu-C-peptide levels following subcutaneous implantation of human islet cell (hu-IC) grafts containing 2–4 × 106 cells (solid lines for Alg-encapsulated and dotted lines for nonencapsulated preparations). Data from nontransplanted control mice are illustrated by the gray lines. All data are expressed as means ± SD. Significance of differences was calculated by one-way ANOVA with Tukey post hoc test, comparing nonencapsulated and encapsulated implants (*P < 0.05, **P < 0.01), encapsulated implants and nontransplanted controls (††P < 0.01, †††P < 0.001), and encapsulated implants in subcutis and peritoneal cavity (‡‡‡P < 0.001).
The increasing plasma p-C-peptide levels for subcutaneous Alg-pp-IC were associated with a decrease in basal glycemia (2-h fast) from PT week 8 onwards to levels measured in pigs (from 114 ± 12 to 86 ± 4 mg/dL) (19) (Fig. 2) and a more rapid glucose clearance than in controls (Supplementary Fig. 1). These effects on glycemia appeared later in recipients of the lower cell dose. They were associated with a suppression of the m-C-peptide response to glucose and a 50% reduction in pancreatic insulin content (Table 1). These data indicate that porcine β-cells control glucose clearance from PT week 10 onwards. Since capsules could be quantitatively retrieved from the subcutis, we directly assessed the FBM that achieved glucose control during the subsequent 10 weeks. This study was conducted on implants with the lower cell dose and involved analysis of the two FBM components, i.e., β-cell number and β-cell functional state.
Suppression of mouse pancreatic β-cells by Alg-pp-IC implants
| PT week . | Plasma C-peptide 15-min post–glucose load (ng/mL) . | Pancreas insulin content (µg/organ) . | |||
|---|---|---|---|---|---|
| Recipient . | Control . | Recipient . | Control . | ||
| p-C-peptide | m-C-peptide | m-C-peptide . | . | . | |
| 4 | 0.7 ± 0.4 | 0.8 ± 0.3 | 0.8 ± 0.2 | ||
| 10 | 3.5 ± 2.5 | 0.2 ± 0.2**† | 1.4 ± 0.8 | ||
| 16 | 6.1 ± 2.6 | 0.1 ± 0.2**†† | 1.0 ± 0.2 | ||
| 20 | 5.1 ± 1.8 | 0.1 ± 0.1**†† | 1.1 ± 0.5 | 16.3 ± 6.7‡‡ | 34.4 ± 6.9 |
| PT week . | Plasma C-peptide 15-min post–glucose load (ng/mL) . | Pancreas insulin content (µg/organ) . | |||
|---|---|---|---|---|---|
| Recipient . | Control . | Recipient . | Control . | ||
| p-C-peptide | m-C-peptide | m-C-peptide . | . | . | |
| 4 | 0.7 ± 0.4 | 0.8 ± 0.3 | 0.8 ± 0.2 | ||
| 10 | 3.5 ± 2.5 | 0.2 ± 0.2**† | 1.4 ± 0.8 | ||
| 16 | 6.1 ± 2.6 | 0.1 ± 0.2**†† | 1.0 ± 0.2 | ||
| 20 | 5.1 ± 1.8 | 0.1 ± 0.1**†† | 1.1 ± 0.5 | 16.3 ± 6.7‡‡ | 34.4 ± 6.9 |
Data are mean ± SD for recipients of Alg-pp-IC grafts (1 × 106 cells [subcutaneously]) (n = 10–14) and controls (n = 4–9). Statistical difference in plasma m-C-peptide levels was calculated by one-way ANOVA with Tukey post hoc test:
**P < 0.001, graft recipients vs. corresponding control mice;
†P < 0.01 and
††P < 0.001, graft recipients beyond PT week 4 vs. PT week 4. Significance of difference between pancreatic insulin content of graft recipients and corresponding control calculated by Student t test:
‡‡P < 0.001.
Establishment of FBM Involves Increase in Porcine β-Cell Number Within Subcutaneous Alginate Microcapsules
At PT week 20, >90% of subcutaneous implants appeared as a flat sheet in which microcapsules were layered within loose connective tissue with closely associated capillaries (Fig. 1C). The capsules contained larger aggregates than at start (cross-sectional surface 3,564 ± 3,490 vs. 1,345 ± 1,739 µm2, P < 0.0001), almost entirely composed of insulin- and glucagon-positive cells (Fig. 1D), with a higher ratio of insulin- over glucagon-positive cells (13.0 ± 4.5 vs. 1.6 ± 0.2 at start, P < 0.001). Occasionally (<10% of implants) the microcapsules floated in a seroma without connective tissue and capillaries; these animals consistently presented lower plasma p-C-peptide levels; they were excluded from further analysis.
Subcutaneous capsules were quantitatively retrieved from the sheets (79 ± 9% recovery of implanted number) and examined for their content. Both cell number and percent β-cells were twofold higher than in the grafts, together indicating a fourfold increase in β-cell number (Table 2), almost entirely accounting for the increase in total cell number. α-Cell number had decreased by 50%, contributing to a fourfold decrease in their proportion. No cell loss was measured during the first PT week; instead, the increase in β-cell number started early and proceeded up to PT week 10. This increase can be attributed to replication of β-cells: the percent Ki67-positive β-cells was high at the time of implantation and declined over 10 weeks to a level that stabilized at 2–3% (Table 2). When capsules were placed in the peritoneal cavity, no increase in cell number was detected and cellular composition (44 ± 4% β-cells and 28 ± 3% α cells) was similar to that at start (42 ± 4% β-cells and 27 ± 3% α cells, P > 0.05) (Fig. 1B and F).
Cellular composition of Alg-pp-IC implants (106 cells) following transplantation
| PT week . | n . | β-Cells . | α-Cells . | ||||
|---|---|---|---|---|---|---|---|
| Number per capsule . | % of total cells . | % Ki67+ . | Insulin content (ng/103 β-cells) . | Number per capsule . | % of total cells . | ||
| 0 | 3–12 | 510 ± 134 | 42 ± 4 | 8.1 ± 1.3 | 7.1 ± 3.3 | 327 ± 79 | 27 ± 3 |
| 1 | 5 | 831 ± 203 | 52 ± 3** | ND | 12.1 ± 2.9 | 464 ± 135 | 29 ± 4 |
| 5 | 3–4 | 1,180 ± 318 | 65 ± 4*** | 5.4 ± 0.3** | 22.8 ± 7.1* | 282 ± 118 | 16 ± 5*** |
| 10 | 3–4 | 2,228 ± 390*** | 67 ± 8*** | 2.6 ± 1.0*** | 32.8 ± 18.7*** | 518 ± 241 | 15 ± 6*** |
| 20 | 6–12 | 1,935 ± 543*** | 81 ± 5*** | 2.8 ± 0.6*** | 30.5 ± 7.8*** | 162 ± 63** | 7 ± 2*** |
| PT week . | n . | β-Cells . | α-Cells . | ||||
|---|---|---|---|---|---|---|---|
| Number per capsule . | % of total cells . | % Ki67+ . | Insulin content (ng/103 β-cells) . | Number per capsule . | % of total cells . | ||
| 0 | 3–12 | 510 ± 134 | 42 ± 4 | 8.1 ± 1.3 | 7.1 ± 3.3 | 327 ± 79 | 27 ± 3 |
| 1 | 5 | 831 ± 203 | 52 ± 3** | ND | 12.1 ± 2.9 | 464 ± 135 | 29 ± 4 |
| 5 | 3–4 | 1,180 ± 318 | 65 ± 4*** | 5.4 ± 0.3** | 22.8 ± 7.1* | 282 ± 118 | 16 ± 5*** |
| 10 | 3–4 | 2,228 ± 390*** | 67 ± 8*** | 2.6 ± 1.0*** | 32.8 ± 18.7*** | 518 ± 241 | 15 ± 6*** |
| 20 | 6–12 | 1,935 ± 543*** | 81 ± 5*** | 2.8 ± 0.6*** | 30.5 ± 7.8*** | 162 ± 63** | 7 ± 2*** |
Data are mean ± SD for grafts with 106 cells at start. ND, not determined. Significance of differences with values at start was calculated by one-way ANOVA with Tukey post hoc test:
*P < 0.05;
**P < 0.01;
***P < 0.001.
In situ analysis confirmed that the cell content of subcutaneous capsules at PT week 20 entirely consisted of endocrine cell aggregates, as marked by their synaptophysin positivity (Fig. 1E). Vimentin-positive cells were localized at their outside periphery; they were not associated with giant cells or dense fibrosis and were thus considered to be mild interstitial reactivity to the biomaterial. We also noticed endocrine cell aggregates in the vicinity of some capsules (Fig. 1E); their absence at PT weeks 1 and 5 suggests that they result from an outgrowth from capsules rather than from nonencapsulated tissue at the start.
Functional State of Porcine β-Cells Following Their Expansion in Subcutaneous Alginate Microcapsules
At PT week 20, encapsulated porcine β-cells exhibited the same insulin-synthesizing capacity as encapsulated human pancreatic β-cells with similar net glucose effect (Table 3); the high proportion of newly synthesized proinsulin over total protein (65% at 10 mmol/L glucose) expresses the predominance of β-cell hormone production in intracapsular activities. Their insulin secretory function rapidly responded to increases or decreases in glucose concentration, as did that of encapsulated human β-cells, but the increase above basal was significantly lower unless glucagon was added to the medium (Fig. 3). However, the net stimulatory effect of this combination represented only 14% of that by encapsulated human pancreatic β-cells (3 ± 2 vs. 21 ± 10 pg insulin/103 β-cells ⋅ min, P < 0.001). This lower release rate is not attributable to lower cellular insulin reserves; on the contrary, cellular insulin content was almost threefold higher than that in encapsulated human β-cells (30.5 ± 7.8 vs. 11.3 ± 4.9 ng insulin/103 β-cells, P < 0.001), and fourfold higher than at start (Table 2), reaching levels that are measured in freshly isolated adult rat β-cells (18). Electron micrographs illustrate that porcine β-cells at PT week 20 contain a higher cytoplasmic density of secretory vesicles than at start (Fig. 4A and B); these exhibit the same ultrastructural characteristics as those in human pancreatic β-cells (Fig. 4C), however, with a higher proportion of granules with lower electron density and narrower halo, considered to represent an immature stage.
Insulin synthetic capacity of Alg-encapsulated β-cells
| . | β-Cell purity . | Insulin synthesis . | ||||
|---|---|---|---|---|---|---|
| % of all cells . | pg/103 β-cells ⋅ h . | % total protein synthesis . | ||||
| . | 0–2.5 mmol/L glucose . | 10 mmol/L glucose . | Net glucose effect . | 0–2.5 mmol/L glucose . | 10 mmol/L glucose . | |
| Alg-pp-IC PT week 20 | 79 ± 4 | 7.0 ± 2.2 | 56.3 ± 14.4*** | 51.3 ± 16.0 | 36 ± 7 | 65 ± 5*** |
| Cultured Alg-hu-IC | 33 ± 9 | 14.3 ± 7.2 | 46.7 ± 19.5* | 32.4 ± 16.2 | 27 ± 13 | 51 ± 11** |
| . | β-Cell purity . | Insulin synthesis . | ||||
|---|---|---|---|---|---|---|
| % of all cells . | pg/103 β-cells ⋅ h . | % total protein synthesis . | ||||
| . | 0–2.5 mmol/L glucose . | 10 mmol/L glucose . | Net glucose effect . | 0–2.5 mmol/L glucose . | 10 mmol/L glucose . | |
| Alg-pp-IC PT week 20 | 79 ± 4 | 7.0 ± 2.2 | 56.3 ± 14.4*** | 51.3 ± 16.0 | 36 ± 7 | 65 ± 5*** |
| Cultured Alg-hu-IC | 33 ± 9 | 14.3 ± 7.2 | 46.7 ± 19.5* | 32.4 ± 16.2 | 27 ± 13 | 51 ± 11** |
Data are mean ± SD of Alg-pp-IC retrieved from subcutaneous implants at PT week 20 (n = 7–9) compared with those in Alg-hu-IC preparations before transplantation (n = 3). Significance of differences between values at low and high glucose concentration calculated by one-way ANOVA with Tukey post hoc test:
*P < 0.05;
**P < 0.01;
***P < 0.001.
Glucose responsiveness of insulin release by Alg-encapsulated prenatal porcine islet cells during perifusion of preparations before transplantation (dotted line) and following retrieval at PT week 20 (solid line) (n = 6–from study with 106 cells at start). Secretory profiles are compared with those of Alg-hu-IC before transplantation (gray line) (n = 4). Insulin secretion is expressed as pg insulin/103 β-cells and as percent of basal release (2.5 mmol/L glucose). Plotted values represent means ± SD. For each preparation, the stimulatory effect of increases in glucose concentration (*P < 0.01, **P < 0.001) and of addition of glucagon (††P < 0.001) was examined by one-way ANOVA with Tukey post hoc test. For each of the glucose stimulatory conditions, release by porcine β-cells was significantly lower than that by human β-cells; this was no longer the case in the presence of glucagon.
Glucose responsiveness of insulin release by Alg-encapsulated prenatal porcine islet cells during perifusion of preparations before transplantation (dotted line) and following retrieval at PT week 20 (solid line) (n = 6–from study with 106 cells at start). Secretory profiles are compared with those of Alg-hu-IC before transplantation (gray line) (n = 4). Insulin secretion is expressed as pg insulin/103 β-cells and as percent of basal release (2.5 mmol/L glucose). Plotted values represent means ± SD. For each preparation, the stimulatory effect of increases in glucose concentration (*P < 0.01, **P < 0.001) and of addition of glucagon (††P < 0.001) was examined by one-way ANOVA with Tukey post hoc test. For each of the glucose stimulatory conditions, release by porcine β-cells was significantly lower than that by human β-cells; this was no longer the case in the presence of glucagon.
Electron microscopy of β-cells in Alg-pp-ICs before transplantation (A) and at posttransplant week 20 (B) compared with β-cells in Alg-hu-ICs before transplantation (C). β-Cells are identified by the presence of secretory vesicles with dense granule core and wide clear halo; vesicles with pale granules and a narrower halo have been shown to contain more unprocessed hormone and therefore considered as immature (38,39). Porcine β-cells at PT week 20 (B) exhibit a higher cytoplasmic density of secretory vesicles than those at start (A) or those in human islet cell preparations (1.8 ± 0.6 vs. 1.2 ± 0.6 and 0.9 ± 0.5 per µm−2, respectively; P < 0.001). Their proportion of mature granules is higher than that at start but lower than that in human β-cells (58 ± 17 vs. 40 ± 16 and 75 ± 15%, respectively; P < 0.001) (one-way ANOVA with Tukey post hoc test). Scale bar = 2 µm.
Electron microscopy of β-cells in Alg-pp-ICs before transplantation (A) and at posttransplant week 20 (B) compared with β-cells in Alg-hu-ICs before transplantation (C). β-Cells are identified by the presence of secretory vesicles with dense granule core and wide clear halo; vesicles with pale granules and a narrower halo have been shown to contain more unprocessed hormone and therefore considered as immature (38,39). Porcine β-cells at PT week 20 (B) exhibit a higher cytoplasmic density of secretory vesicles than those at start (A) or those in human islet cell preparations (1.8 ± 0.6 vs. 1.2 ± 0.6 and 0.9 ± 0.5 per µm−2, respectively; P < 0.001). Their proportion of mature granules is higher than that at start but lower than that in human β-cells (58 ± 17 vs. 40 ± 16 and 75 ± 15%, respectively; P < 0.001) (one-way ANOVA with Tukey post hoc test). Scale bar = 2 µm.
Inability of Adult Human Pancreatic Islet Cells to Establish Sustained FBM in Subcutaneous Alginate Microcapsules
Nonencapsulated human islet cell implants in the subcutis failed to achieve plasma hu-C-peptide levels >0.5 ng/mL, while Alg-hu-IC implants achieved levels >2 ng/mL during the first 10 weeks PT, which was higher than levels of Alg-pp-IC implants during that period (Fig. 2). However, levels in Alg-hu-IC recipients progressively declined during the subsequent 10 weeks, whereas those of porcine cell implants increased. No decrease in basal glycemia to human levels was observed—at variance with the reduction observed with porcine implants. The lower efficacy of encapsulated human cell implants cannot be attributed to a lower β-cell number and purity in their grafts; at start, their capsules contained a similar β-cell number and similar insulin content (11.3 ± 4.9 vs. 7.1 ± 3.3 ng insulin/103 porcine β-cells, P > 0.05) but with virtually no β-cells in proliferative activity (<0.5% Ki67+), which contrasts with the high proportion in porcine grafts (8%). Moreover, human β-cell number per capsule decreased by 71% over 20 weeks (689 ± 208 to 246 ± 20, P < 0.01), together with a decrease in percent living cells (80 ± 5 to 42 ± 18%, P < 0.01), while porcine β-cell number increased fourfold in capsules with >90% viable cells. Microscopic analysis of the capsular content indicated fewer and smaller endocrine cell aggregates than at start and much debris (Fig. 1G and H). Insulin content of residual human β-cells was not significantly different from start values (18.0 ± 8.8 versus 11.3 ± 4.9 ng/103 β-cells, P > 0.05), whereas porcine β-cell insulin content had increased fourfold.
Discussion
This study demonstrates that subcutaneous implants of Alg-pp-ICs can establish a FBM that achieves and maintains metabolic control in mice over a 20-week period. Normal NOD/scid recipients were selected; their basal glycemia is higher than that in pigs (114 ± 21 vs. 86 ± 4 mg/dL) (19), so they can be considered a mildly hyperglycemic model for porcine β-cell implants, as recently reported for human β-cell implants (17). Exposure to this higher glycemia did not prevent formation of a porcine FBM that, from PT week 10 onwards, corrected this hyperglycemic state to levels that are normal for the porcine glucostat. During the subsequent 10 weeks, this porcine FBM was responsible for glucose-induced β-cell responses in the recipients as shown by the plasma p-C-peptide levels in the presence of marginally low and unresponsive m-C-peptide levels. A glucose tolerance test indicated a more rapid glucose clearance than in control mice.
The delay in establishing a FBM with metabolic control is attributable to the time needed for the prenatal islet cells to develop functional implants. This was also the case when these cells were implanted under the kidney capsule before their ability to correct streptozotocin-induced hyperglycemia (7,20). A delay was also seen with porcine neonatal islet cell grafts, although these can be further differentiated before implantation (21,22). The current study shows that differences in initial cell dose influence the duration of this delay, which is another argument for using standardized quality-controlled start preparations to evaluate outcome of implants.
The ability to quantitatively retrieve subcutaneous capsules for quantitative and qualitative analysis of their cellular content allowed a direct assessment of the FBM that was developed to establish this metabolic control. Number and functional state of encapsulated β-cells were determined—two key determinants of FBM and its role in β-cell replacement (8). At PT week 20, the capsules were virtually pure in endocrine islet cells as at the start but now with larger aggregate diameter and higher proportion of β-cells. β-Cell number per capsule had increased fourfold, which is attributable to β-cell replication, in particular during the first 10 weeks PT, initiated by a high percentage of β-cells in proliferative activity at start; such growth was also seen in nonencapsulated prenatal porcine islet cell implants (7,23). We did not observe cells that were double positive for insulin and glucagon but cannot exclude a contribution by α-cell transdifferentiation to β-cells. α-Cell number did not increase, leading to an increase in the proportion of β-cells within the aggregates (from 42 to 81%). This high percentage may be species related, as it characterizes islets in the adult porcine pancreas (81 ± 6% β-cells, our measurements and ref. 24). It can also result from influences in the subcutaneous microenvironment.
Over 20 weeks PT, encapsulated prenatal porcine β-cells gained properties of adult human β-cells. Their secretory activity rapidly responded to changes in glucose concentration, while they were unresponsive at start. Release kinetics during perifusion were similar to those of encapsulated human adult islet cells, both presenting a 1- to 2-min delay in first-phase insulin release compared with nonencapsulated human cell preparations. This delay can thus be attributed to the capsule; since it was measured under in vitro flow conditions, it is not necessarily indicative of the delay in vivo. The insulin secretory amplitude of porcine β-cells was, however, significantly lower. This was not attributable to a lower glucose sensitivity or lower cellular insulin reserve in comparison with encapsulated human islet cell aggregates. It might reflect a functionally state that has not fully matured. It could also result from an inhibitory effect within the intracapsular microenvironment, such as an imbalance between insulin’s suppressive and glucagon’s amplifying influences on glucose-induced insulin release (18,25). The high β–to–α-cell ratio and the correction by addition of glucagon are compatible with the latter mechanism.
Porcine prenatal β-cells can thus increase in number and functionally differentiate in Alg microcapsules placed under the skin of mice. The current study shows that this results in formation and preservation of a metabolically adequate FBM when injecting 4 × 105 β-cells distributed as, on average, 500 β-cells per capsule. Twenty weeks later, the particles contained, on average, fourfold more β-cells, bringing the total for the implant site to 1.6 × 106 β-cells, which is threefold higher than the number in the normal mouse pancreas (26). This FBM formation in the implants was associated with a progressive rise in plasma p-C-peptide responses to glucose leading to amplitudes >5 ng/mL that were maintained up to PT week 20, as was metabolic control. It is conceivable that a lower initial cell dose is also effective, possibly requiring a longer time to achieve metabolic effects in line with their earlier appearance for grafts with threefold more cells at start. Graft efficacy may also vary with the implant site. When Alg-pp-IC grafts were injected in the peritoneal cavity, they failed to develop a FBM with relevant in vivo outcome (plasma p-C-peptide <0.5 ng/mL), indicating that tissue integration is needed for increasing β-cell number and functional state in prenatal porcine β-cell implants. Embedding the porcine prenatal islet cells in Alg did not reduce their outcome in the subcutis—on the contrary, upon injection nonencapsulated, functional β-cells were formed, but the rise in plasma p-C-peptide occurred later and was not accompanied by a lowering of glycemia. Further studies should now examine efficacy of this combination in immune-competent mice, comparing measures to protect against innate and immune reactivity such as size, shape, and Alg type of the capsules (27,28). Modifications should also prevent outgrowth of cells. Outgrowth from Alg capsules has previously been described for other cell types and models and attributed to ruptures in the matrix possibly caused by proliferating cells (29,30). We observed outgrowth from PT week 10 onwards with 2% high M Alg capsules (present data), but also with 3.3% high M Alg, with 10 instead of 1 mmol/L barium during chelation or with the high guluronic acid Alg variant (unpublished observations). Assessment of in vivo capsule stability thus requires longer follow-up periods than usually adopted.
Direct assessment of FBM in encapsulated porcine cell implants, together with measurements of its indirect marker, plasma p-C-peptide, and indices for its metabolic control, will be useful to evaluate modifications to improve outcome in immune-(in)competent recipients. It should also allow comparison of efficacy of the present porcine prenatal islet cell implants in the subcutis with that in previous reports on Alg-encapsulated fetal, neonatal, or adult porcine islet cell implants in subcutis, peritoneal cavity, or other sites (9–13,31–34); this is, however, not possible because of insufficient graft and implant information in these studies.
Alg-encapsulated human adult islet cells also established a FBM in subcutaneous implants as shown by plasma hu-C-peptide levels >2 ng/mL over 10 weeks PT. This was not the case for nonencapsulated implants, indicating benefit of the Alg matrix for survival and function of human β-cells in this site, independently of a protection against immune reactivity. This benefit, however, did not prevent a 70% decline in β-cell number over 20 weeks, which contrasts with the fourfold increase in β-cell number in capsules containing prenatal porcine islet cells. This opposing outcome is attributable to differences in β-cell functional state: human adult β-cells in the graft do not exhibit signs of proliferative activity and do not survive well in the subcutaneous space, possibly caused by their higher susceptibility to its hypoxic microenvironment (35–37). It is thus far unknown whether this is also the case in intraperitoneal capsules. We previously showed that intraperitoneal Alg-hu-IC implants in mice resulted in plasma hu-C-peptide levels over 5 weeks PT similar to those in the present subcutaneous implants; the β-cell dose was twofold higher at start and not determined after this period or beyond (14). Plasma hu-C-peptide levels were followed longer for implants of nonencapsulated human islet cells under the kidney capsule; they also showed a decline after PT week 10 (17). Since such decline was not observed for β-cell implants formed by porcine prenatal islet cells or by human stem cell–derived pancreatic progenitor cells, we propose that early-stage β-cells survive and adapt better than adult β-cells to the microenvironment of extrahepatic sites.
Article Information
Acknowledgments. The authors thank their collaborators at the Diabetes Research Center, Vrije Universiteit Brussel, for technical and administrative support, the team of the Beta Cell Bank for providing (non)encapsulated human pancreatic islet cell preparations, the team of Beta Cell NV for providing encapsulated porcine islet cells, and the team of Drs. Frans Gorus and Ilse Weets at the Clinical Chemistry department for conducting the peptide assays.
Funding. The present work has been supported by grants from JDRF (17-2013-293) and the Flemish Government (IWT130138). I.D.M., T.R., K.G.S., G.M.S., F.V.H., Z.L., D.J.-T.T., B.K., and D.G.P. are members of the Beta Cell Therapy Consortium, which is supported by the European Union and JDRF.
Duality of Interest. D.G.P. is scientific advisor to Beta Cell NV. P.T. is employed by Beta Cell NV. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. I.D.M. collected and analyzed data and drafted the manuscript. T.R., K.G.S., G.M.S., F.V.H., and Z.L. contributed to data collection or analysis and revised the manuscript. P.T., D.J.-T.-T., and B.K. participated in planning the study and revised the manuscript. D.G.P. designed the study, supervised data analysis, and edited the manuscript. D.G.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.